Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.

- SKU
- LAC1008130
- Category
- Uncategorized
FAQs
What is the retail return or exchange policy on this item?
Unopened retail units are eligible for refund or exchange within 30 days of receipt. Initiate a return through your order history or call our retail support team, and we will email a prepaid return label within one business day.
How fast will my order arrive and which warehouse will ship it?
Orders release within 24 hours and ship from the LAC warehouse closest to your ZIP (Chicago, Dallas, or Los Angeles). Most retail customers receive delivery in 1–3 business days with full tracking updates.
Can I qualify for discounts or auto-refill pricing?
Yes. Add two or more cases, enroll in Subscribe & Save, or share your monthly usage and our retail team will apply bundle pricing instantly—no contracts required.
Which payment methods are supported for retail checkout?
We accept all major credit and debit cards, digital wallets (Apple Pay, Google Pay), Buy Now Pay Later, and HSA/FSA cards. ACH and wire options are also available for larger storefront purchases.
What kind of support do I get after purchasing?
Retail customer care is available 7 days a week by phone, chat, or email. Need product guidance, reorder help, or claim assistance? Text or call 844-LAC-6091 and we will respond within an hour.
C.R. Bard FOL0102 - StatLock Foley Stabilization Device, Tricot Anchor Pad, for Silicone Catheter, Adult, 25/BOX
Product Description C. R. Bard FOL0102 – StatLock Foley Stabilization Device, Tricot Anchor Pad, for Silicone Catheter, Adult, 25/BOX C. R.
C.R. Bard FOL0102 – StatLock Foley Stabilization Device, Tricot Anchor Pad, for Silicone Catheter, Adult, 25/BOX
C.R. Bard FOL0102 StatLock Foley Stabilization Device
StatLock Stabilization Devices are a more effective alternative to tape in helping improve clinical outcomes, quality of care and economic efficiency. The StatLock Foley stabilization device accommodates latex 8-22 Fr. and silicone 8-26 Fr. catheters for the ultimate in versatility. Available in adult and pediatric sizes.
C.R. Bard FOL0102 StatLock Foley Stabilization DeviceFeatures and Benefits
- Reduce Foley catheter movement
- Minimize accidental catheter dislodgements
- Maximize patient comfort by:
- Eliminating circumferential compression
- Alleviating traction of urethral catheters
- The StatLock Foley Stabilization Device sets new, worldwide standard for Foley catheter stabilization.
- Featuring a patented swivel retainer for enhanced patient comfort and convenience.
- Available in adult and pediatric sizes for most latex and silicone catheters.
- Releasable, lock-tight securement.
StatLock Device Retainer Design
- Lock-tight design stabilizes catheter to prevent pistoning and accidental dislodgement.
- Swivel design allows catheter movement with the patient without exerting a pull force on the catheter.
- Releasable design allows for easy cleaning of patient.
- Accommodates both 2-way and 3-way latex 822 Fr. and silicone 626 Fr. catheters for the ultimate in versatility.
StatLock Device Anchor Pad Design
- Available in breathable latex-free tricot fabric for added patient comfort.
- Available in both pediatric and adult sizes, giving you the versatility needed for effective Foley catheter stabilization.
- Designed to be easier to use than tape and circumferential leg straps.
- Unique alcohol-soluble adhesive is gentle on the skin.
C.R. Bard FOL0102 StatLock Foley Stabilization Device Indications for Use
The StatLock device is a stabilization device for compatible catheters.
Warnings and Precautions of C.R. Bard FOL0102 StatLock Foley Stabilization Device
- Do not use the StatLock device where loss of adherence could occur, such as with a confused patient, diaphoretic or non-adherent skin, or when the access device is not monitored daily.
- Observe universal blood and body fluid precautions and infection control procedures, during application and removal of the StatLock device.
- Minimize catheter manipulation during application and removal of the
C.R. Bard FOL0102 StatLock Foley Stabilization Device Application Technique
Prep
- 1.Place Foley catheter into retainer. Directional arrow should point towards catheter tip, and balloon inflation arm should be next to the clamp hinge.
- 2. Close lid, being careful to avoid pinching the catheter.
- 3. Identify securement site by laying the device retainer on the front of the thigh, leaving 1 inch of catheter slack between insertion site and the StatLock device retainer.
- 4. After placing the StatLock Stabilization device off to the side, cleanse and degrease the securement site with alcohol per hospital policy. Let skin dry.
- 5. Apply skin protectant, in direction of hair growth, to area larger than securement site. Allow to dry completely (10-15 seconds).
- 6. Using permanent marker, write initials and date of application on the StatLock device anchor pad.
NOTE: Always secure catheter into the StatLock device retainer before applying adhesive pad on skin.
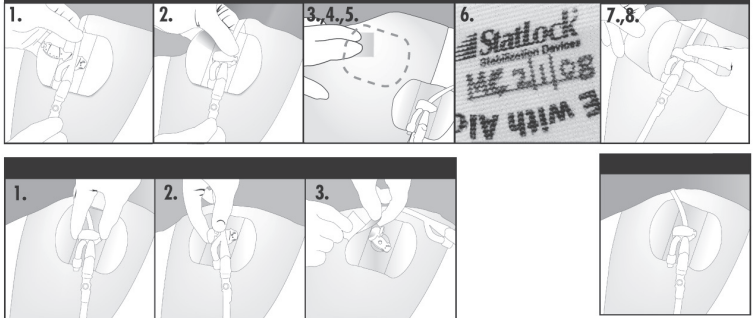
Place and Peel
- 7. Align the StatLock Stabilization Device over securement site leaving 1 inch of catheter slack. Make sure leg is fully extended.
- 8. While holding the retainer to keep the pad in place, peel away paper backing, one side at a time and place tension-free on skin.
Daily Maintenance
- The StatLock device should be assessed daily and changed when clinically indicated, at least every seven days.
- If pad becomes soiled, wash with soap/water, saline or hydrogen peroxide. Do not use alcohol or prepackaged bathing systems, which could lead to early lifting.
- If showering/bathing, cover with plastic wrap or waterproof dressing.
- Conduct skin assessment prior to application and repeat daily per facility protocol.
- Use clinical judgment on the removal of the StatLock Stabilization device if the patient experiences any fluid shifts that may interfere with skin integrity
Removal Technique
Disengage
- 1. Open retainer by pressing release button with thumb, then lift to open.
- 2. Remove Foley catheter from the StatLock device.
Dissolve
- 3. Wipe the edge of the pad using at least 5-6 alcohol pads until a corner lifts. Then continue to stroke undersurface of pad with alcohol to dissolve adhesive pad away from skin.
Do not pull or force pad to remove.
Device Characteristics of C.R. Bard FOL0102 StatLock Foley Stabilization Device
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as “Not made with natural rubber latex”: | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Pricing is bespoke—request a quote (valid for the next 2 hours).

Volume pricing, managed services, and consignment programs available through LAC Health procurement.
Contract & blanket PO support
GHX • Workday • SAP integrations
Delivery & Shipping
Check ETA for your dock in seconds.
Free standard shipping on orders over $75
Express and white-glove freight options available
Let’s architect a resilient supply programme together.
Invite our procurement strategists into your planning room and we’ll deliver a clinically-aligned sourcing roadmap—complete with compliance, telemetry, and logistics tuned to your realities.
Customer reviews
What procurement leaders and clinical teams are saying after deploying this SKU.
Arrived cold-chain validated with serialized lots pre-registered in our ERP. Teams aligned rapidly thanks to LAC’s transition kit and consultative launch.
