Product Description
3M 1650 – DRESSING, IV, TEGADERM, SECURAL, 4″X6 18″, 100 EA/CS, 4 BX/CS
3M Tegaderm I.V. Transparent Film Dressing with Border 1635
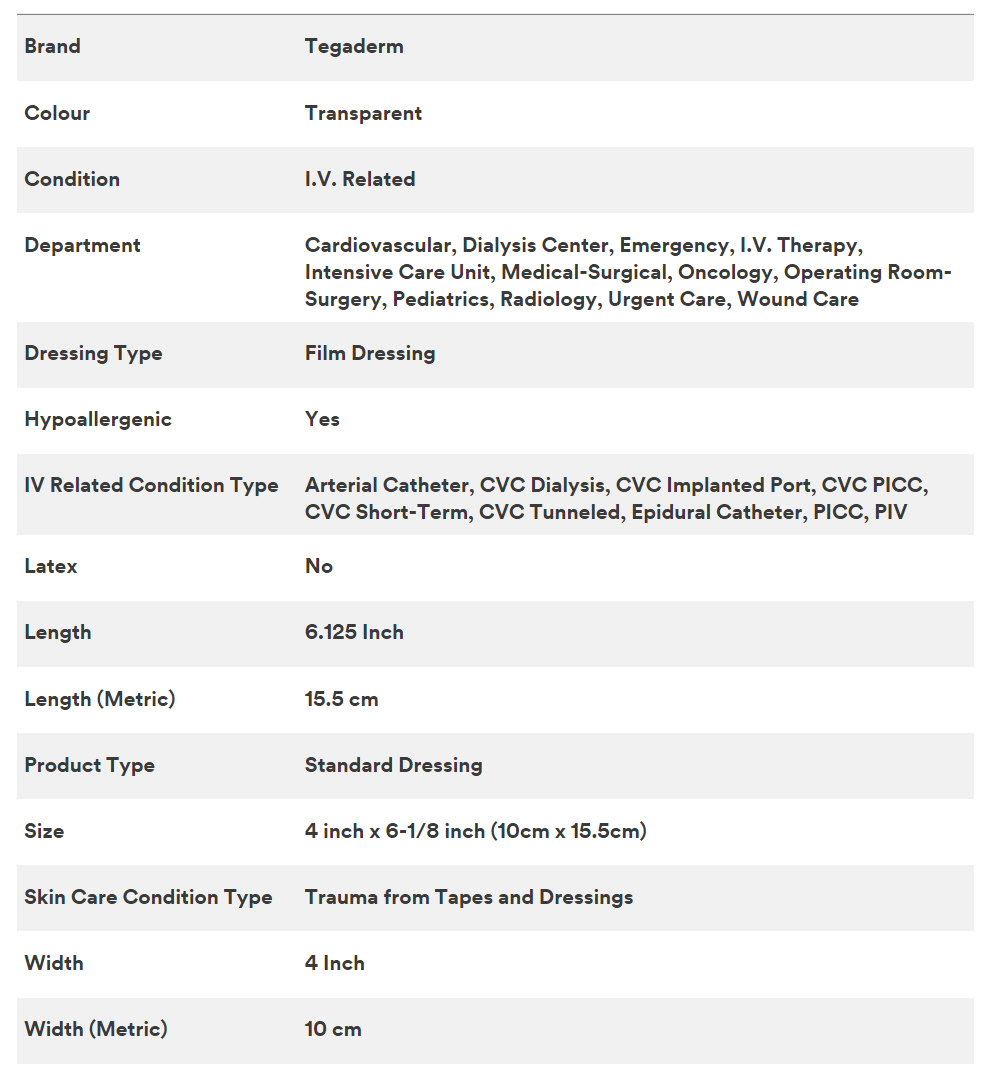
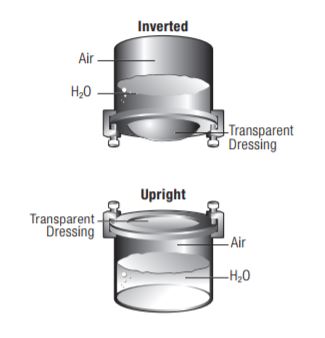
Tegaderm Film consists of a thin film backing with a non-latex, hypoallergenic adhesive. Tegaderm Film with Border is notched and reinforced with soft cloth tape to provide a better seal around catheters and other devices. The dressing is breathable, allowing good oxygen and moisture vapor exchange. It is waterproof and impermeable to liquids, bacteria, and viruses. An intact dressing protects the site from outside contamination.
In vitro testing shows that the film of Tegaderm and Tegaderm HP dressings provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- Provides a waterproof, sterile barrier to external contaminants including liquids, bacteria and viruses.
- Transparent dressing allows for continuous visibility to the I.V. insertion site.
- Adhesive is gentle on skin.
- Dressing flexes with skin for greater patient comfort.
- Picture-frame delivery makes accurate placement easy.
Features and Benefits
Versatile – One Product to Satisfy Many Clinical Situations
Tegaderm dressings can be used to protect I.V. sites, enhance wound healing, prevent skin breakdown, and protect clean, closed surgical incisions. Tegaderm dressings are available in many sizes, shapes and application styles to meet a wide variety of needs. The frame allows the dressings to be tailored for special applications, when desired. Application systems of most other transparent dressings do not allow for this customization.
Easy to Apply – Unique Frame Delivery System
Application of Tegaderm dressing is intuitive and quick, making it easy to remember and easy to teach. It is especially convenient for patient self-care. Tegaderm dressing minimizes application time and saves dressing waste and costs. The frame delivery system provides maximum control of the thin film for rapid application of even the largest dressings. The unique “picture-frame” allows precise and secure placement of the dressing every time. If the adhesive surface accidentally touches itself, the dressing can be separated and applied, eliminating wasted dressings. Tegaderm dressing is also available in a first-aid style delivery system for the health care professional who prefers this application method.
Gentle Adhesive – Just the Right Balance in Adhesive Strength
Tegaderm dressings are made with a hypoallergenic, latex-free adhesive that is gentle to the skin, yet securely holds catheters and other devices in place. Tegaderm dressing provides good initial adhesion without building to excessive levels over time. Even for dressings left in place for extended periods, the risk of patient discomfort and skin trauma is minimal when the dressing is properly removed.
Breathable – Lets Oxygen In and Moisture Vapor Out
The breathability of Tegaderm dressings allows moisture vapor and gas exchange, which is essential to maintain normal skin function under the dressing. Patients can wear Tegaderm dressings for extended periods of time, with minimal risk of skin irritation or maceration, and without excessive proliferation of skin flora.
Waterproof, Sterile Barrier – Impervious to Liquids, Bacteria and Viruses
Tegaderm dressing acts as a barrier to protect the I.V. site or wound from external contaminants such as bacteria, viruses, blood and body fluids. Because Tegaderm dressings are waterproof, patients may bathe, shower or swim, if the dressing is completely sealed around the catheter or wound. Tegaderm dressing is sterile and remains so as long as the outer package is intact. Do not resterilize by gamma, steam, or E-beam.
Conformable – Flexes with Skin for Greater Patient Comfort
3M Tegaderm Transparent Film Dressing conforms to body contours, stretches easily, and prevents stress on the skin when the patient moves. It protects skin and bony prominences from abrasion, and allows the patient to move easily. Tegaderm dressing is comfortable to wear and presents a flat profile. The special shapes of Tegaderm dressings conform easily on difficult-to-dress areas, such as jugular and PICC insertion sites, and sacral wounds.
Enhanced Wound Healing – Improved Outcomes and Patient Comfort
Tegaderm dressing seals in natural wound fluid to maintain a moist environment, which has been shown to enhance the healing process. It prevents scab formation and dehydration of the wound bed, which can occur with conventional dry dressings. Tegaderm dressing provides a wound environment that allows epithelial cells to migrate easily across the wound surface, reducing pain caused by wound dehydration, and increases patient comfort.
Transparent – Allows Wounds and I.V. Sites to Be Easily Monitored
The transparency of Tegaderm dressing provides complete visibility of the site during application. It also allows continuous monitoring of the I.V. site or wound without disturbing or removing the dressing. This visualization eliminates unnecessary dressing changes and saves nursing time.
Fewer Dressing Changes – Increased Patient Comfort
With Tegaderm dressing, fewer dressing changes mean improved patient comfort and the risk of skin trauma from repeated adhesive removal is reduced. For I.V. care, fewer dressing changes result in less catheter manipulation, and reduced exposure to potential outside contaminants. For wound applications, the longer wear time of Tegaderm dressing allows the wound to remain undisturbed, thereby preventing disruption of the healing process.
Tegaderm dressings are breathable, sterile, transparent and waterproof, and provide a barrier to external contaminants.
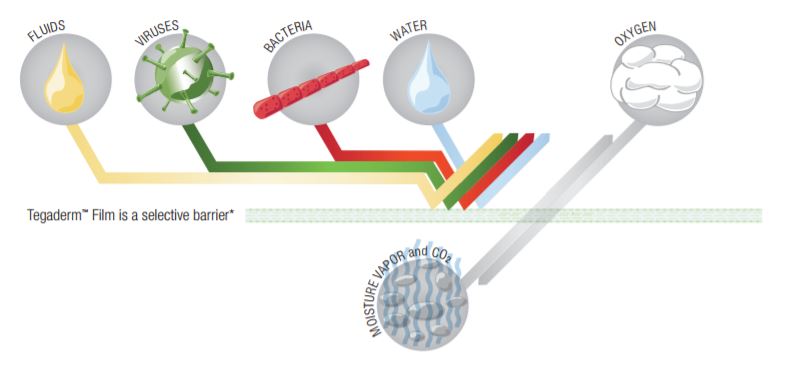
Physical Properties/Definitions
Semi-Occlusive (Semi-Permeable)
Tegaderm and Tegaderm HP Transparent Film Dressings are made of semi-permeable films. They can be thought of as selective filtersthey are occlusive to liquids, bacteria, and viruses;* yet water vapor, oxygen, and carbon dioxide can easily be exchanged. Tegaderm and Tegaderm HP Film dressings are breathable. The breathability of a material is generally described in terms of oxygen and moisture vapor transmission rates (MVTR). Both rates are determined by the amount of gas that travels through the dressing in a given period of time, under specific conditions of temperature and humidity.
MVTR (Moisture Vapor Transmission Rate)
Moisture vapor transmission rate (MVTR) is the measurement of water vapor diffusion through a material. Two laboratory test methods are commonly used to measure MVTR. The results of these two tests are often used to compare transparent dressings for I.V. use. However, they do not represent real life conditions, and numerous variables can impact the results. This raises the question of whether laboratory test data for MVTR can accurately predict dressing performance in clinical practice.
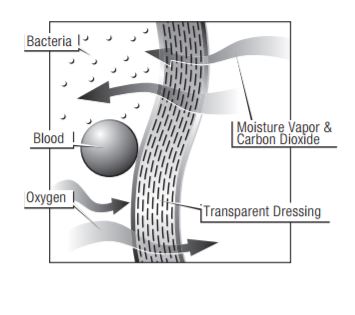
The inverted beaker test produces higher numbers with greater variability. These variances are seen within samples of the same dressing, as well as among different products. This inconsistency occurs because the films can stretch and swell due to the water pressure against the test dressing, increasing the surface area measured. The MVTR values produced by the upright method are lower and more consistent among different products, and within samples of the same dressing. Because the liquid does not come in contact with the film in this test method, stretch and swell are not factors in the results.
Aside from the test method chosen, many other variables can dramatically affect moisture vapor transmission rates.
- Volume of liquid in the test beaker (generally 1050 ml)
- Type of liquid medium (water, saline)
- Concentration of substances in the liquid (salt, proteins)
- Environmental conditions (temperature, humidity)
MVTR bench tests are generally performed under tightly controlled temperatures and low relative humidity. In clinical settings, where temperatures and humidity vary considerably from typical test conditions, MVTR numbers will be much different than those produced in the laboratory. For example, under conditions of high humidity, moisture vapor transmission will proceed at a much slower rate.
A third, less common method, uses computerized evaporimetry to measure moisture handling properties of transparent dressings. This instrument records actual evaporation through the film on skin, and moisture build-up underneath the dressing. When moisture vapor transmission is measured with this device, dressings with significant differences in bench MVTRs show no significant difference in actual moisture accumulation on the skin.
Suggested Applications
- To cover and protect I.V. catheter sites
- Acute and chronic wounds
- Skin protection
- Feeding tubes
- Superficial partial thickness burns
- Stage I or II pressure ulcers
- Autolytic debridement facilitated by the moist wound healing environment
- Protective eyelid coverings
- Post tattoo application and removal
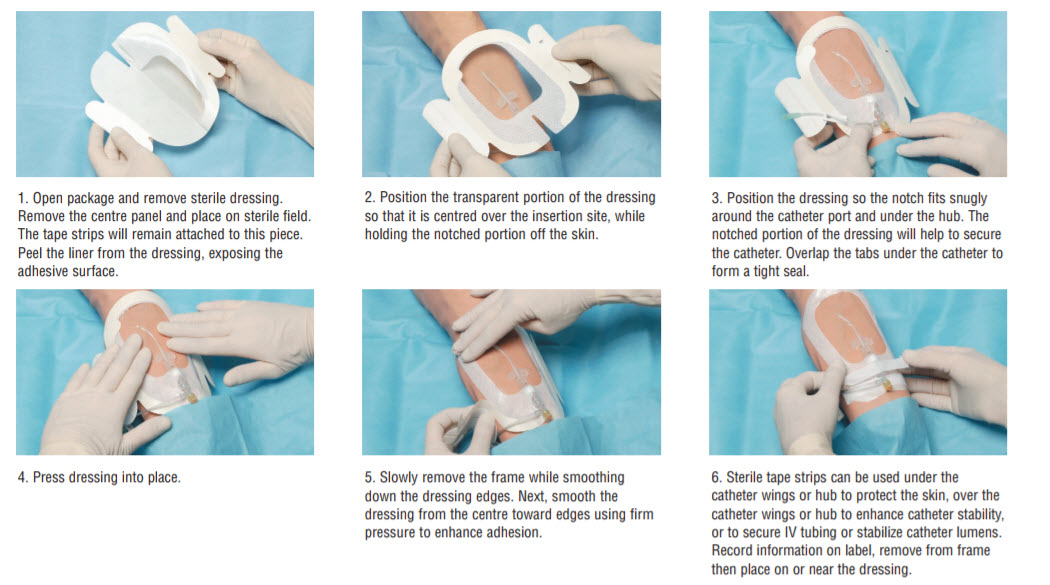
Application
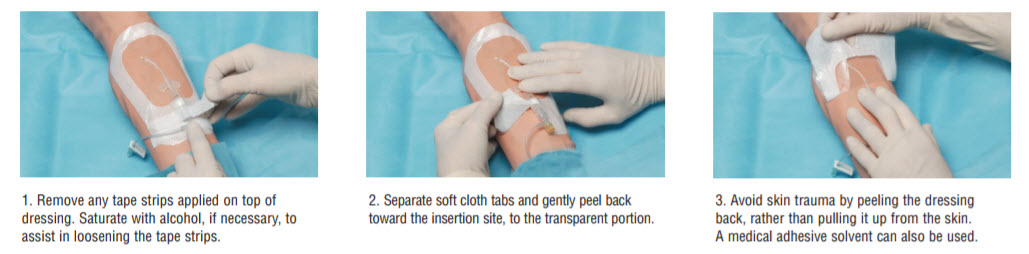
Removal
Specifications