Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.
Loading...
Loading...
LAC Health leverages cutting-edge AI and machine learning to optimize procurement, predict demand, and streamline your healthcare operations. Partner with us to unlock next-generation efficiency.
500k+
SKUs Available
AI
Demand Forecasting
97%
On-Time Delivery
24hrs
Response Time
Unopened retail units are eligible for refund or exchange within 30 days of receipt. Initiate a return through your order history or call our retail support team, and we will email a prepaid return label within one business day.
Orders release within 24 hours and ship from the LAC warehouse closest to your ZIP (Chicago, Dallas, or Los Angeles). Most retail customers receive delivery in 1–3 business days with full tracking updates.
Yes. Add two or more cases, enroll in Subscribe & Save, or share your monthly usage and our retail team will apply bundle pricing instantly—no contracts required.
We accept all major credit and debit cards, digital wallets (Apple Pay, Google Pay), Buy Now Pay Later, and HSA/FSA cards. ACH and wire options are also available for larger storefront purchases.
Retail customer care is available 7 days a week by phone, chat, or email. Need product guidance, reorder help, or claim assistance? Text or call 844-LAC-6091 and we will respond within an hour.
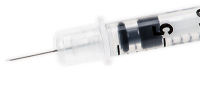
Product Description BD 328466 – Syringe . 5cc 30gx1/2″ U100 Ultra-Fine Insulin 100/Bx, 5 BX/CA BD Ultra-Fine Insulin Syringes 1/2mL 12. 7mm (1/2″) 30G 328466 – BD Insulin Syringes with the BD Ultra-Fine needle 12.
BD 328466 Ultra-Fine Needle is a 30-gauge, 12.7mm (1/2″) syringe needle engineered to provide comfort, accuracy and ease in injecting. Reduces the risk of a painful injection in the muscle.
| Hub Color | Clear |
| Hub Material | Polypropylene |
| Hub Type | Rounded |
| Needle Gauge | 30 G |
| Needle Gauge (m) | 0.30 mm |
| Needle Length (in.) | 1/2 in. |
| Needle Length (m) | 12.70 mm |
| Needle Tip Type | 3-bevel |
| Needle Type | Insulin |
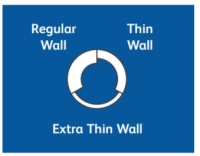
| Needle Wall Type | Thin wall |
| Syringe Scale | 1 mL graduations |
| Sterile | Sterilized product |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Hypodermic syringe and needle with shielding mechanism |
| Sterilization Method | Gamma radiation |
| Latex Statement | Not made with natural rubber latex |
| Disposable | Disposable product |
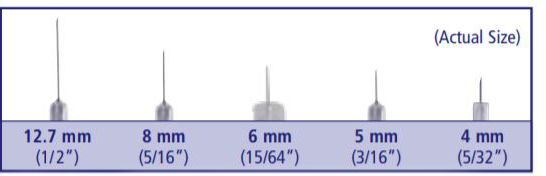
Insulin needles come in different lengths. BD Original Needles are 12.7 mm (1/2″) long, and BD Short Needles are 8 mm (5/16″) long. The BD Nano Needle 4mm (3/16″) and BD Mini Needle 5mm (5/32″) are available only in pen needles. For a comfortable injection experience, shorter needle lengths are recommended and preferred.
The higher the gauge, the thinner the needle. For example, 32G is thinner than a 31G needle. Insulin needles are available in many gauges (G), or thicknesses. The higher the gauge, the thinner the needle. For example, a 32 G needle is actually thinner and more comfortable than a 29 G needle.
Regular Wall: This is the most common wall thickness. The thickness of the steel wall allows a good flow rate, and minimizes flexing when the needle is inserted into a vial stopper or patient.
Most commonly found in insulin and ‘tuberculin’ syringes. Permanently attached needles, also known as integral needles, reduce the amount of medication waste and allow accurate mixing of different medications into one syringe.
BD Products comply with the regulatory requirements of the region in which these are sold and manufactured.
All products which are labeled as #sterile# and released for sale by BD are certified to be sterile per EN 556-1 Sterilization of Medical Devices as long as the package is unopened and undamaged. This product is sterilized via Cobalt 60 – Irradiation. Sterilization cycle development/validation is performed to 10-6 SAL in accordance with current ISO 11137 guidelines.
This product has been evaluated in accordance with ISO 10993 “Biological Evaluation of Medical Devices”, and complies with all relevant sections.
All products which are labeled as non-pyrogenic and released for sale by BD have been tested per United States Pharmacopeia (USP) chapter (85)- Bacterial Endotoxins Test and meets limits as specified in chapter 161- Transfusion and Infusion Assemblies and Similar Medical Devices.
Representative production samples are collected and inspected in accordance with current applicable product specifications. Inspection records are reviewed and signed off by qualified personnel for product release. The released devices meet applicable BD product specification(s).
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | No |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Proper injection technique is essential to improve consistency in medication delivery and optimize glycemic control. It includes factors such as:
Proper syringe disposal will help to:
| Packaging Level | Shelfpack | Case | Each |
| Quantity | 100 | 500 | 1 |
| Length | 171.45 mm | 431.8 mm | |
| Width | 139.7 mm | 184.15 mm | |
| Height | 84.15 mm | 152.4 mm | |
| Weight | 339.996 g | 1.92 kg | 4.073 g |
$42.53

Volume pricing, managed services, and consignment programs available through LAC Health procurement.
Contract & blanket PO support
GHX • Workday • SAP integrations
Delivery & Shipping
Check ETA for your dock in seconds.
Free standard shipping on orders over $75
Express and white-glove freight options available
Invite our procurement strategists into your planning room and we’ll deliver a clinically-aligned sourcing roadmap—complete with compliance, telemetry, and logistics tuned to your realities.
What procurement leaders and clinical teams are saying after deploying this SKU.
Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.
Arrived cold-chain validated with serialized lots pre-registered in our ERP. Teams aligned rapidly thanks to LAC’s transition kit and consultative launch.