Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.
Loading...
Loading...
LAC Health leverages cutting-edge AI and machine learning to optimize procurement, predict demand, and streamline your healthcare operations. Partner with us to unlock next-generation efficiency.
500k+
SKUs Available
AI
Demand Forecasting
97%
On-Time Delivery
24hrs
Response Time
Unopened retail units are eligible for refund or exchange within 30 days of receipt. Initiate a return through your order history or call our retail support team, and we will email a prepaid return label within one business day.
Orders release within 24 hours and ship from the LAC warehouse closest to your ZIP (Chicago, Dallas, or Los Angeles). Most retail customers receive delivery in 1–3 business days with full tracking updates.
Yes. Add two or more cases, enroll in Subscribe & Save, or share your monthly usage and our retail team will apply bundle pricing instantly—no contracts required.
We accept all major credit and debit cards, digital wallets (Apple Pay, Google Pay), Buy Now Pay Later, and HSA/FSA cards. ACH and wire options are also available for larger storefront purchases.
Retail customer care is available 7 days a week by phone, chat, or email. Need product guidance, reorder help, or claim assistance? Text or call 844-LAC-6091 and we will respond within an hour.
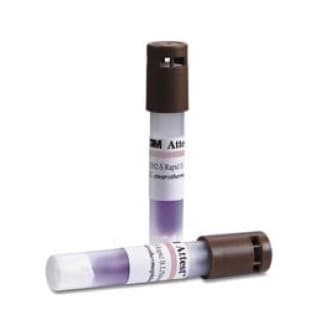
The ‘Attest Rapid Steam Biological Indicator 1292-S’ is a reliable and convenient tool for validating and monitoring steam sterilization processes. It provides accurate results in just 3 hours and is suitable for use in industrial steam sterilization applications.
The 3M Attest 1292-S Rapid Readout Biological Indicator for Steam is a fast (3 hour readout), reliable, and convenient biological indicator for validating and monitoring steam sterilization processes when used in conjunction with the 3M Attest 293 or 290 Auto-readers. The Attest 1292-S may be used as a component of the quality system for a steam sterilization process that ensures that medical devices or pharmaceutical products are processed per the manufacturers specifi cations and comply with the requirements of national and international sterilization standards. The self-contained design of the Attest 1292-S reduces testing time and eliminates the potential for cross-contamination associated with the culturing of conventional spore strips. The fl uorescent readout can provide confi rmation of an acceptable cycle after only 3 hours of incubation.
The Attest 1292-S provides fast, reliable results that allow the potential for inventory optimization with the assurance of using a biological indicator system for validating and monitoring the sterilization process.
The Attest 1292-S has been validated as an effective biological monitor for 132? (270?) vacuum-assisted and 121? (250?) gravity steam sterilization processes under specifi c test conditions. The Attest 1292-S could also be used to monitor other industrial steam sterilization processes, such as 134? (273?), with appropriate testing and validation by the end user. The suitability of the Attest 1292-S for monitoring any industrial steam sterilization process must be verifi ed and documented by the end user.
Note: The 3M Attest Biological Indicator 1292-S for Steam Sterilization is only compatible with the 3M Attest 390 Auto-reader.
The following information is provided with the Attest 1292-S biological indicators and provides specifi c use instructions.
Identifi cation and Expiration Date: Each biological indicator will be labeled with a lot code that also provides the expiration date information. The format of this code is noted below:
YYYY-MM XX
Y denotes the Year the product will expire; M denotes the Month the product will expire (last day of that month); XX is a 3M lot specifi c code. For example:
2010-03 JK
represents a product with production code JK that will expire on March 31, 2010. The entire lot code (8 characters) must be used to describe or document the product lot. Biological indicators that are beyond their expiration date should not be used.
Inspection: Damaged biological indicators may produce erroneous results. Indicators can be damaged in shipping, or in handling (e.g. during placement into, or extraction from, a product or a process challenge device); damage by pallet handling before or after processing). Careful inspection of the biological indicator both before and after processing (before crushing the ampoule) is critical. (Note: Please follow safety precautions described in WARNING section when inspecting indicators that have been exposed to the sterilization process).
Carefully inspect each biological indicator for:
Any damaged or suspect biological indicators should be discarded. Any results obtained from damaged biological indicators should be considered suspect.
|
Biological Indicator System |
Rapid |
|
Brands |
Attest |
|
Cap Color |
Brown |
|
Catalog Number |
1292-S |
|
Factory ISO Certification |
No ISO Certification information available |
|
Incubation Period |
3 Hour |
|
Inner Packs per Case |
2 Cartons per Case |
|
Product Type |
Biological Indicator |
|
Sterilization Method |
Steam |
|
Total Units per Case |
600 per Case |
|
Units per Inner Pack |
300 per Carton |
$2,744.57
$2,889.02
Save 5% ($144.45)
Volume pricing, managed services, and consignment programs available through LAC Health procurement.
Contract & blanket PO support
GHX • Workday • SAP integrations
Delivery & Shipping
Check ETA for your dock in seconds.
Free standard shipping on orders over $75
Express and white-glove freight options available
Invite our procurement strategists into your planning room and we’ll deliver a clinically-aligned sourcing roadmap—complete with compliance, telemetry, and logistics tuned to your realities.
What procurement leaders and clinical teams are saying after deploying this SKU.
Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.
Arrived cold-chain validated with serialized lots pre-registered in our ERP. Teams aligned rapidly thanks to LAC’s transition kit and consultative launch.