Moog Medical 340-4133 – NON-DEHP TUBING W/ NON-VENTED BAG SPIKE AND NEEDLESS INJ POINT, 20 PER/CS
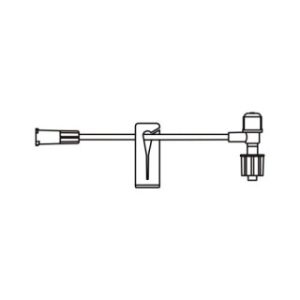
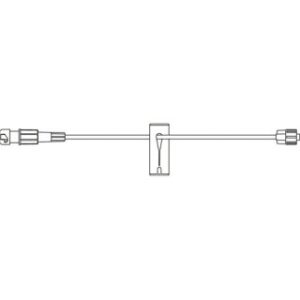
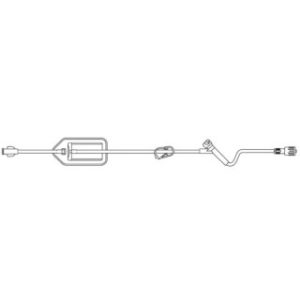
340-4130-V – 98″ / 4.2 mL Non-DEHP Microbore Tubing with Non-Vented Bag Spike and Needleless Injection Port (Positive Pressure)

Non-vented bag spike, microbore tubing with free-flow protection, slide clamp, ULTRASITE injection site 6 in. above distal end, distal male luer lock connector. Priming volume: 4.6 mL, Length: 98 in. (248.9 cm)
All administration sets designed for use with Moog’s Curlin line of infusion pumps include anti-free flow feature that prevents inadvertent free flow of the infusion. This unique and patented anti-free flow device also allows the intentional priming of the set by gravity, thereby reducing the set-up time. In addition to the standard administration sets, Moog features administration sets with an anti-siphon valve included for additional anti free-flow protection All ambulatory infusion administration sets can be used with the Moog 6000 CMS, PainSmart IOD, and 4000 CMS electronic infusion pumps.

Preparing Medication for Infusion/Changing IV Bags


Spiking the fluid container, gravity priming the set and using the slide clamp to close the tubing.
|
|
|
|
Curlin Medical Administration set “Integral Flow-Stop” with breakaway tab |
Intentionally Opening the “Integral Flow-Stop” |
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as “Not made with natural rubber latex”: | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |








Reviews
There are no reviews yet.