Cook Medical G53704 – SET, STENT, URETERAL, SOFT, 5FX18CM, US-600-R, EACH

Universa Soft Ureteral Stent and Positioner
Stent and positioner – (No wire guide)
The Universa Soft Ureteral Stents and Stent Sets are flexible tubular devices made from radiopaque polyurethane. The stents are available with or without hydrophilic coating. Each end of the Universa Soft Ureteral Stent consists of a curled, tapered pigtail. Sideports are placed on each end of the stent, including on the pigtails of the stent. A braided or monofilament tether for repositioning and removal of the device is located on the proximal pigtail of the stent. Radiopaque graduation marks are located on the stent to increase fluoroscopic visualization during stent advancement and placement.
The Universa Soft Ureteral Stent Set includes
- Radiopaque double pigtail stent
- Stainless steel wire guide
- Radiopaque stent positioner with radiopaque tip
- Pigtail straightener
| Order Number | Reference Part Number | Fr | Length (cm) |
| G53704 | US-600-R | 6.0 | 22-32 |
Experience Universa
Universa stents provide temporary internal drainage from the ureteropelvic junction to the bladder. Universa products have premium features and the everyday advantage of a full product line. Consolidated part numbers enable streamlined ordering and provide cost savings. The Universa Soft stent offers a softer feel for patient comfort.
Features and Benefits

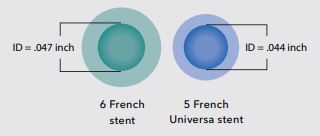
Comparison
Enlarged cross-section comparison of a typical 6 Fr stent and 5 Fr Universa stent
Intended Use
The Universa Soft Ureteral Stents and Stent Sets are used for temporary internal drainage from the ureteropelvic junction to the bladder. Ureteral stents have been used to relieve obstruction in a variety of benign, malignant and post-traumatic conditions. The stents may be placed using endoscopic, percutaneous or open surgical techniques.
Precautions
- The potential effects of phthalates on pregnant/nursing women or children have not been fully characterized and there may be concern for the reproductive and developmental effects.
- The Universa Soft Stents must not remain indwelling more than six months. If the patients status permits, the stent may be replaced with a new stent.
- These stents are not intended as permanent indwelling devices.
- The tether should be removed if the stent is to remain indwelling longer than 14 days.
- Do not force components during removal or replacement. Carefully remove the components if any resistance is encountered.
- A pregnant patient must be more closely monitored for possible stent encrustation due to calcium supplements.
- Improper handling can seriously weaken the stent. Acute bending or overstressing during placement may result in subsequent separation of the stent at the point of stress after a prolonged indwelling period.
- Angulation of the wire guide or stent should be avoided. Use of a 0-degree scope lens is recommended. Scopes larger than 21.0 French are suggested.
- Individual variations of interaction between stents and the urinary system are unpredictable.
- Ureteral stents should be checked periodically for signs of encrustation and proper function. Periodic checks of the stent by cystoscopic and / or radiographic procedures are recommended at intervals deemed to be appropriate by the physician in consideration of the individual patients condition and other patient specific factors. The stent is not intended as a permanent indwelling device which should not exceed 180 days.
- Use of the device should be based upon consideration of risk-benefit factors as they apply to your patient.
Potential Adverse Events
Complications of ureteral stent placement are documented. These complications include, but are not limited to:
- Extravasations
- Occlusion
- Migration
- Hemorrhage
- Sepsis
- Perforation of the urinary tract
- Peritonitis
- Encrustation
- Urinary tract infection
- Loss of renal function.
Product Recommendations
If no wire guide is provided with this set, the following is recommended:
- 5.0 French stents accept .035″
- 6.0 French stents accept .038″
- 7.0 French stents accept .038″
- 8.0 French stents accept .038″
MRI Information
Nonclinical testing has demonstrated that the Universa Ureteral Soft Stents are MR Conditional according to ASTM F2503. A patient with this device may be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5 tesla or 3.0 tesla only
- Maximum spatial gradient magnetic field of 1600 gauss/cm (16.0 T/m) or less
- Maximum MR system reported, whole-body-averaged specific absorption rate (SAR) of










Reviews
There are no reviews yet