Baxter 2C7558 – SET IV CHEMOTHERAPY PACLITAXEL DEHP-FREE FLUID PATH PE LINED TUBING NON-VENTED 111IN SLIDE AND ROLLER CLAMP NEEDLE-FREE INJECTION PORT 0.22U FILTER 10GTTS/ML MALE LUER LOCK LATEX-FREE W/U490944R UR103 (48/CS)
Paclitaxel Set, Non-Vented, INTERLINK Injection Site, 0.2 Micron Filter, 10 drops/mL, 107″ (2.7 m)
Paclitaxel Sets
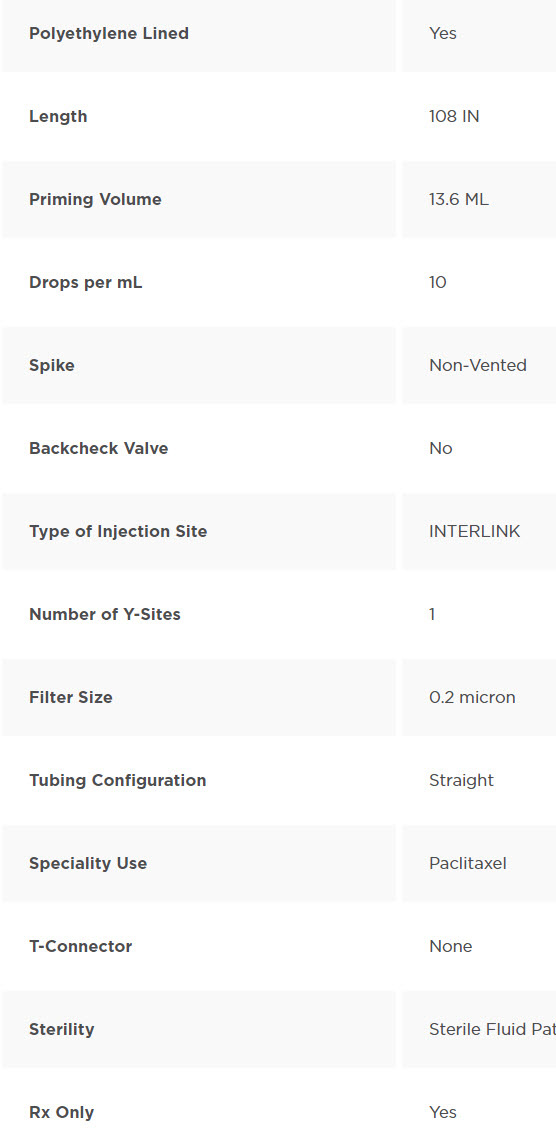
Paclitaxel Set Non-Vented Spike with Polyethylene Lined Tubing and INTERLINK Injection Site, 0.2 Micron Filter, Male Luer Lock Adapter. Approximately 10 drops/mL. Approximate length 108″ (2.7 m). Non-DEHP Pump Segment. Sterile Fluid Path. Fluid path does not contain DEHP; outer layer of polyethylene lined tubing contains DEHP.

Spike is designed so that 10 drops equal approximately 1 mL. Used often by Anesthesiologists in the OR.

Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as “Not made with natural rubber latex”: | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
Contains DEHP
Diethyl hexyl Phthalate, A chemical commonly used in PVC tubing; used to yield clarity and soft pliable tubing.
Product Characteristics
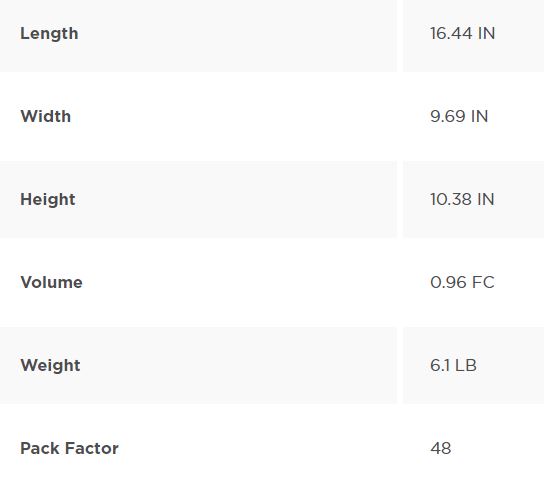
Packing Information







Reviews
There are no reviews yet