Product Description
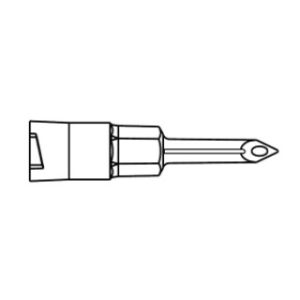
B Braun 4252519-02 – Introcan Safety IV Catheter 22 Ga. x 1 in., FEP, Straight, EACH
B Braun 4252519-02 Introcan Safety IV Catheter 22 Ga. x 1 in., FEP, Straight
Available sterile packaged. Contact representative for information on bulk, non-sterile options. Teflon is a registered trademark of E. I. du Pont de Nemours and Company.
- Teflon catheter material, straight hub
- Not Made With DEHP
- Not Made With Natural Rubber
- Not Made With PVC
Device Characteristics of B Braun 4252519-02 Introcan Safety IV Catheter 22 Ga. x 1 in., FEP, Straight
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): |
No |
| Device labeled as “Not made with natural rubber latex”: | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |




Reviews
There are no reviews yet