B Braun 415110 – Ultrasite Valve Ea, 100 EA/CS
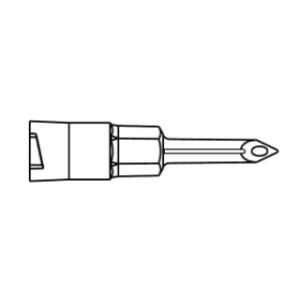
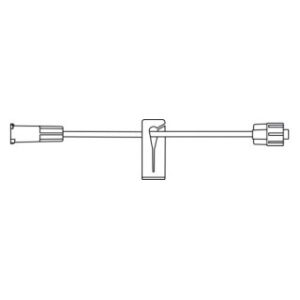
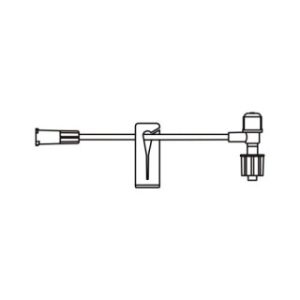
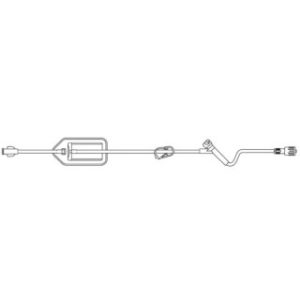
ULTRASITE Luer Access Device
The ULTRASITE Luer Access Device is designed to prevent blood drawbacks when injecting, aspirating or infusing fluids and medications.
The ULTRASITELuer access device is a needleless connector which can be used anywhere a standard injection cap is used and is compatible with leading pre-filled medication systems. Featuring patented Luer Access Device (LAD) technology, which virtually assures compliance by preventing needle access, B. Brauns ULTRASITE LAD is designed to protect both patients and healthcare professionals by reducing accidental needlestick injuries (NSIs) and preventing blood drawbacks when injecting, aspirating or infusing fluids and medication.
Features:
- Quick, effortless Luer access connection.
- High flow promotes rapid fluid delivery.
- Microbial barrier design.
- Easy to clean and disinfect.
- Reduces accidental touch contamination.
- Also available, ULTRASITE Ag Antibacterial Luer Access Device.
Product Identification
- Product ID (Product Code) 0CSU100.
- Reference Number 415110.
- NDC / WSN Number 08021-4151-10.
Safety Data
- BPA Information: Contains BPA.
- DEHP Information: Components Do Not Contain DEHP.
- Latex Information: Components Do Not Contain Natural Rubber.
- PVC Information: Components Do Not Contain PVC.






Reviews
There are no reviews yet